OLABS ∨
解决方案 ∨
雅培(Abbott)公司正在召回Ellipse植入式心脏复律除颤器,原因是其生产过程存在问题缺陷,导致一些铝线部分裸露在外,从而引发了电气故障。此次在美国境内召回的设备中,有31台已经植入患者体内。
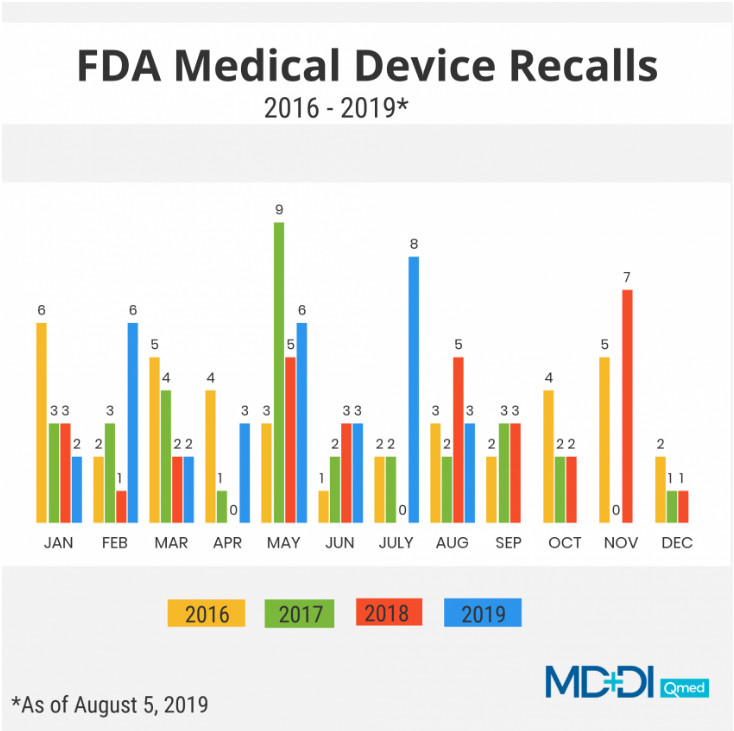
这是2019年美国发生的第33起医疗器械召回事件。考虑到在2017年和2018年这两年里,全年只有32起器械召回事件,而今年才过去三分之二,却已然发生了33起,相比之下,这个数字实属相当多了。(数据来源:FDA;图表制作者:AmandaPedersen)
雅培公司正在召回Ellipse植入式心脏复律除颤器(ICD),原因是其生产过程存在问题缺陷,导致一些铝线部分裸露在外,从而引发了电气故障。
这些ICD除颤器含有未完全绝缘的铝线,容易造成电容器短路。而这类问题对患者的潜在影响可能包括无法为其提供高压治疗。目前还没有可用的方法或程序确保在故障发生之前就能确定哪些设备存在这类问题。
雅培表示,该公司还没发现关于相关已植入体内设备发生此类故障的任何报道。此次召回的设备中,有31台已经植入美国患者体内。根据现有的投诉和医疗器械报告(MDR),要么已更换了相关设备,要么计划更换ICD除颤器发电机。根据所有这些投诉或医疗器械报告,未发生任何患者受伤或其他不良事件,更没有死亡报告。
受此次召回影响的108台Ellipse植入式心脏复律除颤器于2019年4月5日至2019年5月29日期间生产,而其销售时间介于2019年5月6日至2019年6月14日之间。
据FDA数据库显示,今年迄今为止,FDA共报告了33起医疗器械召回事件,而去年同期为14起,且去年全年仅32起。FDA还表示,2017年共报告了32起医疗器械召回事件,而2016年共报告了39起。
有关2016年至2019年这四年各个月份医疗器械行业的召回情况(截至2019年8月5日),请参见上文截图。
FDA最近表示,该机构正在采取新的措施,用于强化和更新关于主动召回公共警告的发布程序以及召回通知程序。
FDA指出,“大多数公司纷纷响应FDA的要求,迅速启动主动召回行动,并与供应链合作伙伴一起开展相关工作,及时将所涉及产品从货架上撤下来,从而防止将其进一步分销出去。”一般来说,一旦发现问题时,相关单位组织就会立即部署召回行动。但是,在有些情况下,为了保护消费者的合法权益,FDA可能需要向市场提供安全建议。
今年实施了医疗器械召回的公司包括以下这些(按从先到后的发生顺序排列):
Abbott、BectonDickinson、Teleflex、GE Healthcare、Hamilton Medical、Edwards Lifesciences、Vyare Medical、Cook Medical、TerumoMedical、Integra Lifesciences、Beckman Coulter Lifesciences、Ethicon、Alpha OmegaEngineering、BrainLab、O-Two Medical Technologies、RVO 2.0、PhysicalControl、Medtronic、Smiths Medical、West Pharmaceutical Services、TerrificCare/Medex Supply、Draeger Medical
英文原文
Abbott's Ellipse Recall Is the 33rd U.S. MedicalDevice Recall This Year
Abbott is recalling the Ellipse implantable cardioverter defibrillators because electrical failures have been identified due to a faulty manufacturing process causing some aluminum wires to be partially exposed. Of the devices being recalled in the United States, 31 have been implanted in patients.
The recall marks the 33rd medical device recall in 2019. That's quite a bit considering that in 2018 and 2017 there were only a total of 32 device recalls each year.(Data Source: FDA; Graph by Amanda Pedersen)
Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical failures have been identified and determined to be due to a faulty manufacturing process causing some aluminum wires to be partially exposed.
ICDs, which contain aluminum wires that are not fully insulated are prone to electrical shorting ofthe capacitor. The potential patient impact could be the inability to deliver high voltage therapy. There iscurrently no available method or procedure to determine which of these devices have this issue prior to failure.
Abbott said it is not aware of any reports of this failure occurring in any affected implanted devices. Of the devices recalled, 31 have been implanted in U.S. patients. The complaints and medical device reports (MDRs) available have either reported that the affected devices have been replaced or are scheduled to be replaced with another ICD generator. None of the complaint or MDRs indicate that any patient harm or adverse events have occurred, and no deaths have been reported.
The 108 Ellipse ICDs affected by this recall were manufactured between April 5, 2019, and May 29, 2019. The devices were distributed between May 6, 2019, and June 14, 2019.
FDA has reported atotal of 33 medical device recalls so far this year compared to 14 device recalls this time last year, and a total of only 32 device recalls the entire year, according to FDA's database. The agency also reported 32 device recalls in 2017, and 39 device recalls in 2016.
See the chart above for a complete month-by-month breakdown of recalls in the medical device sector from 2016 through 2019 (as of Aug. 5, 2019).
FDA recently said it is taking new steps to strengthen and modernize the process for issuing apublic warning about a voluntary recall and for notification of recalls.
"Most companies collaborate with the FDA to rapidly initiate voluntary recalls and work with their supply chain partners to remove the product from shelves to prevent further distribution," the agency noted. "And in general, a recall occurs quickly when the problem is discovered. However, there are situations where the FDA may need to provide safety advice to [the] marketplace to protectconsumers."
Companies that have recalled medical devices this year include (in order from most recent): Abbott, Becton Dickinson, Teleflex, GE Healthcare, Hamilton Medical, Edwards Lifesciences, Vyaire Medical, Cook Medical, Terumo Medical, Integra Lifesciences, Beckman Coulter Lifesciences, Ethicon, Alpha Omega Engineering, Brainlab, O-Two Medical Technologies, RVO 2.0, Physio-Control, Medtronic, Smiths Medical, West Pharmaceutical Services, Terrific Care/Medex Supply, Draeger Medical.
来源:MDDI
原文链接:https://www.mddionline.com/abbotts-ellipse-recall-33rd-us-medical-device-recall-year
整理翻译:奥咨达
如果你有寻找医疗器械孵化,
那就与我们取得联系吧!
您可以填写右边的表格,让我们了解您的服务需求,这是一个良好的开始,我们将会尽快与你取得联系。当然也欢迎您给我们写信或是打电话,让我们听到你的声音!
24小时免费咨询热线:
400-6768632
您想参观OLABS创新工厂,
那就与我们取得联系吧!
您可以填写右边的表格,让我们了解您的服务需求,这是一个良好的开始,我们将会尽快与你取得联系。当然也欢迎您给我们写信或是打电话,让我们听到你的声音!
24小时免费咨询热线:
400-6768632