OLABS ∨
解决方案 ∨

3月13日,加拿大卫生部(Health Canada)公布了两个公共数据库,这是其在公开药品和医疗器械安全性和有效性临床信息上迈出的第一步。
目前,药品和器械数据库各显示一条信息:2016年发给CSL Behring Canada(一家专业的生物制药公司)的一则合规通知,以及去年12月授予西门子医疗系统有限公司(Siemens Healthcare GmbH)的一项上市批准。
就计划公开临床信息的相关事宜进行磋商后,加拿大卫生部从去年4月起敲定了最终指南,为如何实施其临床信息公开发布行动奠定了基础。
这份长达32页的指南旨在帮助业界和其他人了解为临床数据公开发布准备临床信息时应遵循的程序、个人信息的保护以及对商业机密信息(CBI)拟议修订的要求。
加拿大卫生部表示,该指南描述了法规所规定的有限而具体的情况,在这些情况下,在药品或医疗器械上市许可申请的临床部分中发现的信息可能在最终监管决定后具有持续的商业价值。这些情况为业界在药品申请中提议修订商业机密信息制定了合格标准。
加拿大卫生部打算在未来的指南文件中具体说明符合条件的医疗器械商业机密信息的类别。目前,加拿大的器械上市许可申请正在根据IMDRF ToC格式进行修改,并将于4月开始实施。与此同时,加拿大卫生部表示,“器械制造商在提出符合加拿大医疗器械法规(CMDR)第43.12(2)节规定的例外情况的修订建议时,应参考第5节的规定。”
在未来四年内,临床信息的公开发布将根据不同类型的申请和产品分阶段进行。对于现有产品,加拿大卫生部还打算根据要求,通过其临床信息门户发布临床信息。
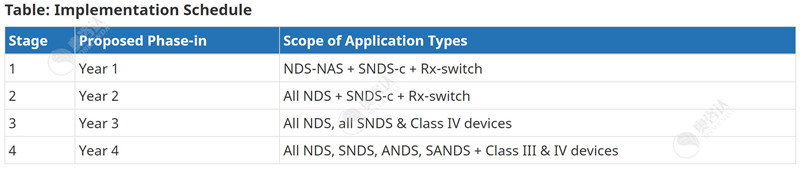
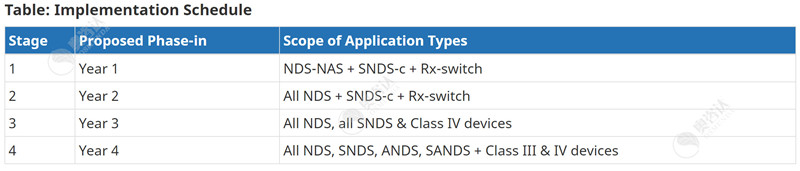
针对主动披露临床信息方面,加拿大卫生部将分四个实施阶段进行。前两阶段只适用于某些药物的申请,而第三及第四阶段则适用于III类和IV类医疗器械的申请。下表1详细列出了法规对药品和医疗器械上市许可申请的适用情况以及信息公开的执行时间,下表2列出了根据申请类型分四个阶段进行主动披露的时间表。

Health Canada Begins Release of Clinical Data on Drugs and Devices
Health Canada launched two public databases on Wednesday as the first step in making clinical information about the safety and effectiveness of drugs and medical devices publicly available.
The drug and device databases currently display just one record for each: a notice of compliance issued to CSL Behring Canada in 2016 and an approval granted to Siemens Healthcare GmbH last December.
Following consultation on Health Canada’s plans to start making clinical information public, the agency finalized guidance from last April that sets the stage for how to implement its Public Release of Clinical Information (PRCI) initiative.
The 32-page guidance is intended to aid industry and others in understanding the procedures to follow in preparing clinical information for public release of the clinical data, the protection of personal information and the requirements for proposed redaction of confidential business information (CBI).
Health Canada said that it has “described limited and specific circumstances, prescribed in regulations, where information found within the clinical component of a drug submission or medical device application may possess ongoing commercial value following the final regulatory decision.” These circumstances set the eligibility criteria for industry to propose redactions of CBI in drug submissions.
The agency intends to specify the eligible CBI categories for medical devices in future guidance. Device applications in Canada are currently being revamped based on the globally agreed upon Table of Contents (ToC) format, with implementation set to start in April. In the meantime, Health Canada said “device manufacturers should refer to Section 5 for guidance in proposing redactions consistent with the exceptions specified in the Medical Device Regulations section 43.12(2).”
The release of clinical information will be a phased-in process for different types of submissions and products throughout the next four years. For existing products, Health Canada also intends to release clinical information, upon request, through the agency’s clinical information portal.
For the proactive disclosure of clinical information, Health Canada will follow four implementation stages. The first two stages will only apply to certain drug submissions, whereas stages 3 and 4 will apply to the publication of Class III and IV medical device applications. The tables below detail the schedule to implement and apply the regulations, followed by the schedule for proactive disclosure by application type based on the four stages.

来源:RAPS
原文链接:https://www.raps.org/news-and-articles/news-articles/2019/3/health-canada-begins-release-of-clinical-data-on-d
整理翻译:奥咨达
奥咨达翻译团队根植于中国,面向全球,专注为医疗器械领域的企业提供专业、高效的翻译解决方案。翻译领域包括医疗器械的研发、注册、临床、上市后监督、营销、管理、培训等,译稿已涵盖医疗器械领域的所有类型。
奥咨达翻译组联系方式:
邮箱:trans@osmundacn.com
如果你有寻找医疗器械孵化,
那就与我们取得联系吧!
您可以填写右边的表格,让我们了解您的服务需求,这是一个良好的开始,我们将会尽快与你取得联系。当然也欢迎您给我们写信或是打电话,让我们听到你的声音!
24小时免费咨询热线:
400-6768632
您想参观OLABS创新工厂,
那就与我们取得联系吧!
您可以填写右边的表格,让我们了解您的服务需求,这是一个良好的开始,我们将会尽快与你取得联系。当然也欢迎您给我们写信或是打电话,让我们听到你的声音!
24小时免费咨询热线:
400-6768632